















Do you want to contribute by writing guest posts on this blog?
Please contact us and send us a resume of previous articles that you have written.
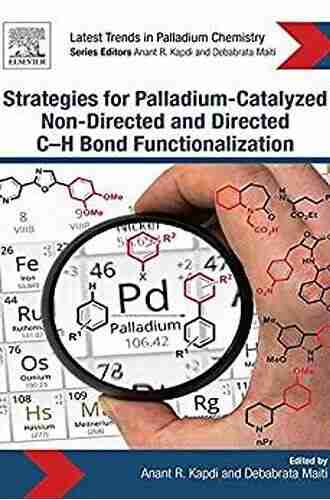
Unlocking the Power of Palladium: Strategies for Palladium Catalyzed Non-Directed and Directed Bond Bond

In recent years, palladium-catalyzed reactions have emerged as some of the most powerful tools in modern organic synthesis. With remarkable versatility and efficiency, these reactions have revolutionized the way chemists create complex molecules. Among the various transformations that can be achieved, palladium-catalyzed directed and non-directed bond formation reactions have gained significant interest due to their utility in the synthesis of pharmaceuticals, agrochemicals, and advanced materials.
Palladium, a transition metal from the platinum group, exhibits unique catalytic properties and plays a vital role in mediating a wide range of chemical reactions. Understanding the strategies for palladium-catalyzed non-directed and directed bond formation can unlock a plethora of possibilities for synthetic chemists.
Non-Directed Bond Formation
Non-directed bond formation, also known as reaction bonding, involves the formation of a new bond between two atoms or groups that are not directly attached to the palladium catalyst. This strategy offers a high level of regio- and stereoselectivity, making it a preferred method for synthesizing structurally complex molecules.
4.7 out of 5
| Language | : | English |
| File size | : | 43762 KB |
| Text-to-Speech | : | Enabled |
| Screen Reader | : | Supported |
| Enhanced typesetting | : | Enabled |
| Print length | : | 447 pages |
| Hardcover | : | 566 pages |
| Item Weight | : | 1.58 pounds |
| Dimensions | : | 6.25 x 1.25 x 9 inches |
One of the most widely used non-directed palladium-catalyzed reactions is the Suzuki-Miyaura coupling. In this reaction, an aryl or vinyl boronic acid reacts with an aryl or vinyl halide, resulting in the formation of a carbon-carbon bond. This transformation has found widespread applications in the synthesis of pharmaceuticals, natural products, and materials.
Another important non-directed bond formation reaction is the Heck reaction. In this process, an aryl or vinyl halide undergoes oxidative addition with palladium, followed by transmetallation with an olefin, and finally, reductive elimination to form a new carbon-carbon bond. The Heck reaction has proven invaluable for the synthesis of a diverse range of compounds, including biologically active molecules and polymers.
Directed Bond Formation
Directed bond formation, also known as regioselective or selective reaction bonding, involves the formation of a new bond between a nucleophilic and electrophilic partner, both directly coordinated to the palladium catalyst. This strategy allows for more control over the regiochemistry of the reaction, leading to specific and predictable product formation.
A prominent example of directed palladium-catalyzed bond formation is the Buchwald-Hartwig amination. In this transformation, an aryl or vinyl halide reacts with an amine, resulting in the formation of a carbon-nitrogen bond. This reaction has become a cornerstone in pharmaceutical synthesis, enabling the late-stage functionalization of complex molecules and facilitating the rapid synthesis of drug candidates.
Furthermore, the Stille coupling is another valuable directed bond formation reaction. It involves the coupling of an organotin compound with an aryl or vinyl halide, resulting in the formation of a carbon-carbon bond. The Stille coupling has been extensively used in natural product synthesis and materials chemistry.
Optimizing Palladium-Catalyzed Reactions
To achieve high yields and selectivity, several factors need to be considered when designing palladium-catalyzed bond formation reactions. Ligand selection plays a crucial role as it can influence the reaction rate, regioselectivity, and catalyst stability. Optimal ligands are often chelating phosphine ligands with bulky substituents to control steric effects and electronic properties.
Reaction conditions, including solvent choice, base selection, and reaction temperature, also impact the success of the reaction. Additionally, the correct choice of palladium precursor and catalytic additives can fine-tune the reactivity and selectivity of the overall process.
Strategies for palladium-catalyzed non-directed and directed bond formation reactions have opened new avenues in organic synthesis, providing a powerful toolset for chemists. The ability to create carbon-carbon and carbon-nitrogen bonds with high efficiency and selectivity has revolutionized the synthesis of complex molecules for various applications.
By understanding the principles behind these reactions and optimizing reaction conditions, synthetic chemists can harness the full potential of palladium catalysis, paving the way for the development of new drugs, materials, and functional molecules.
4.7 out of 5
| Language | : | English |
| File size | : | 43762 KB |
| Text-to-Speech | : | Enabled |
| Screen Reader | : | Supported |
| Enhanced typesetting | : | Enabled |
| Print length | : | 447 pages |
| Hardcover | : | 566 pages |
| Item Weight | : | 1.58 pounds |
| Dimensions | : | 6.25 x 1.25 x 9 inches |
Strategies for Palladium-Catalyzed Non-directed and Directed C-H Bond Functionalization portrays the complete scope of these two aspects of C-H bond functionalization in a single volume for the first time. Featured topics include the influence of palladacyclic systems in C-H bond functionalization (need for newer catalytic systems for better efficiency),mechanistic aspect of the functionalization strategies leading to better systems, and applications of these methodologies to natural product synthesis and material synthesis.
- Addresses the involvement of catalytic systems (palladacycles) for better functionalization of (hetero)arenes to emphasize the need for developing better, more selective systems
- Covers the use of powerful mechanistic tools for understanding and assisting the development of better functionalization strategies
- Discusses the finer aspects of C-H bond functionalization, such as control of regioselectivity with or without directing groups
- Includes a chapter detailing the synthesis of naturally occurring molecules or functional molecules via both pathways for assessing the applicability of the functionalization strategies

 Drew Bell
Drew BellCompulsion Heidi Ayarbe - A Gripping Tale of Addiction...
Compulsion Heidi Ayarbe...

 Guy Powell
Guy PowellThe Cottonmouth Club Novel - Uncovering the Secrets of a...
Welcome to the dark and twisted world of...

 Ira Cox
Ira CoxThe Sociopolitical Context Of Multicultural Education...
Living in a diverse and interconnected world,...

 Jesse Bell
Jesse BellThe Epic Journey of a Woman: 3800 Solo Miles Back and...
Embarking on a solo journey is a...

 Cody Blair
Cody BlairFlorida Irrigation Sprinkler Contractor: Revolutionizing...
Florida, known for its beautiful...

 Walt Whitman
Walt WhitmanUnveiling the Political Tapestry: Life in Israel
Israel, a vibrant country located in the...

 Allan James
Allan JamesLife History And The Historical Moment Diverse...
Do you ever find yourself...

 George Bernard Shaw
George Bernard ShawMiami South Beach The Delaplaine 2022 Long Weekend Guide
Welcome to the ultimate guide for...

 Edison Mitchell
Edison MitchellAn In-depth Look into the Principles of the Law of Real...
The principles of the...

 Caleb Carter
Caleb CarterExclusive Data Analysis Explanations For The October 2015...
Are you preparing for the Law School...

 Alexandre Dumas
Alexandre DumasThe Secret to Enjoying Motherhood: No Mum Celebration of...
Being a mother is a truly remarkable...

 Wesley Reed
Wesley ReedRace Walking Record 913 October 2021
Are you ready for an...
Light bulbAdvertise smarter! Our strategic ad space ensures maximum exposure. Reserve your spot today!
 Adrian WardFollow ·16.7k
Adrian WardFollow ·16.7k Jace MitchellFollow ·12.4k
Jace MitchellFollow ·12.4k Henry JamesFollow ·14.8k
Henry JamesFollow ·14.8k Braden WardFollow ·5.3k
Braden WardFollow ·5.3k Mario BenedettiFollow ·17k
Mario BenedettiFollow ·17k Leo MitchellFollow ·5.1k
Leo MitchellFollow ·5.1k Hamilton BellFollow ·19.6k
Hamilton BellFollow ·19.6k Harrison BlairFollow ·5.7k
Harrison BlairFollow ·5.7k